
Inspection of In-Coming Materials
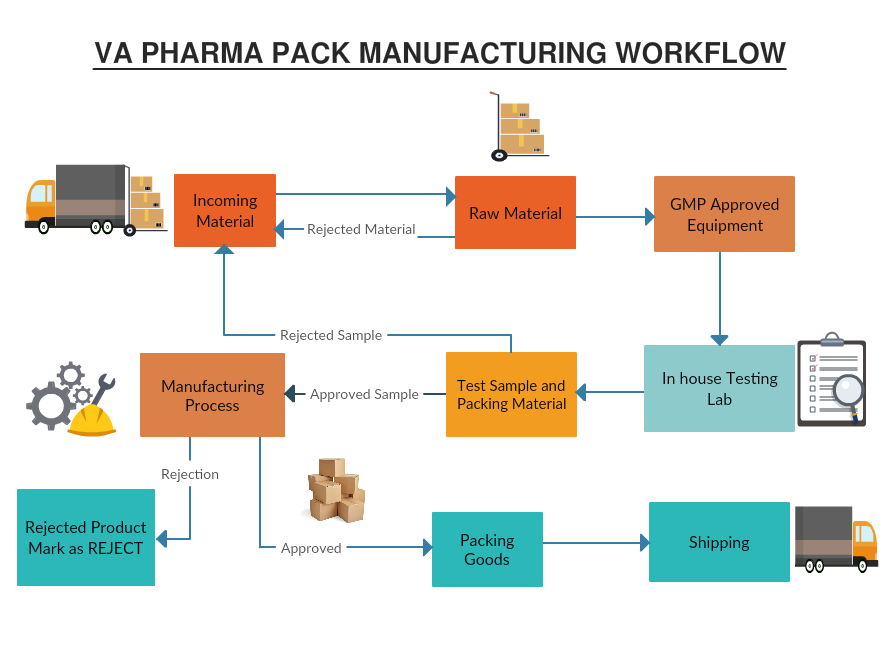
Incoming materials-inspection of raw materials, packaging, and quality as per vendor qualified test report. Quality Assurance and Inspection based on GMP standard. Following of FIFO system. Clear Labelling of rejected and approved material for further clearance to production.
In- Process Inspection
The Production Department is totally responsible to achieve the required standards in quality of finished product. For this purpose, the Quality Assurance Department interacts and assess the Finished Goods. The Quality Assurance Department has full powers to ensure that requisite step of production are followed and quality SOP are maintained.
All raw materials and processing occurs in GMP approved equipment qualified for Food Grade application as per approved technical specification or specified relevant standards.
Only materials as per Technical Specifications approved by Quality Assurance Department are accepted and stored in raw materials stores.
For the incoming materials the stores department records the data in the in-word register. The Quality Assurance Department is informed through in-slip and the incoming material is stored in the receiving inspection area, untill clearance by Quality Assurance Department.
100% inspection or alternatively as per sampling plan laid down in SOP standard are carried out in accordance with the Quality Assurance Plan for each product.
The Quality Assurance Department arranges to send the test samples and packing materials to In-house testing lab for approval. Material Test Certificates on various technical criteria is created – approved material is marked accordingly and cleared by Quality Assurance Department for manufacturing. The rejected raw material and packaging material are marked with REJECTED tags and returned to the supplier / vendor.
The Quality Assurance Team carries out inspection and testing at the stages defined in the Quality Assurance Plan for the product. Tools and instruments calibration and maintenance of test standards. Maintaining test records and inspection results. Inspection report on Equipment and Machinery, production area are followed through before approving for production.
All materials are issued along with a Production team Instructions for manufacturing . Instructions shall contain all the operations and methods in a sequence. Only approved and technically approved quality materials are taken up for further manufacturing process.
Final Inspection
Quality Assurance verifies the dimension, adsorption, material code, printing, sealing and part number etc. on the finished product before acceptance. The Quality Assurance Team carries out the final inspection and tests on the products as per the SOP for the Finished Product. A Final Inspection Report confirms material for dispatch . The final inspection report shall be authorised by Plant Head and Quality Assurance Team
For rejected post productions batches are marked by the Quality Assurance Team with “REJECT” tag and fill-up the Final Inspection Report.
The Quality Assurance Head releases the finished goods for packing only after the stages specified in the SOP have been completed satisfactorily and all the inspection and test reports are available and authorised by him. The accepted material is marked for further packaging with approved marking. The accepted materials are stored in the proper Finished Goods Storage area as per identification mark. The materials are dispatched against a material requisition slip for each specific requirement.
